BOUTIQUEResouces
In the biopharmaceutical field, the accurate analysis and control of Host Cell Proteins (HCPs) are critical links in ensuring the safety and efficacy of drugs. Regulatory authorities worldwide have provisions regarding HCPs, requiring that biopharmaceuticals must undergo analysis and purification to reduce HCPs to acceptable levels.
Regulatory Requirements for Host Cell Proteins (HCPs) by Different Authorities:
· Chinese Pharmacopoeia (2020 Edition), Volume III: Specifies that for CHO cells, residual HCPs must be < 0.05% (equivalent to less than 500 ppm); for E. coli (Escherichia coli), residual HCPs must be < 0.01%.
· United States Pharmacopeia (USP) <1132> Chapter: Stipulates that HCPs in pharmaceuticals should be detected using a highly sensitive method, and their content should be below the limit of detection (LOD) (typically less than 100 ppm, i.e., the HCP content in 1 mg of total protein should be less than 100 ng, or < 0.01%).
· European Pharmacopoeia (EP) 2.6.34: States that in biological products, the content of HCPs should be less than 0.1%.
· International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guidelines: ICH Q6B points out that residual HCPs must be monitored using sensitive and validated effective methods in accordance with ICH guidelines, and their residual amount is generally required to be less than 100 ppm.
· ELISA is the method recommended by pharmacopoeias worldwide for detecting residual HCPs in biological products, as it can determine the total amount of HCPs. Additionally, ELISA is a classic immunological detection method with simple operation. However, ELISA also has limitations in HCP detection: for example, it is impossible to determine which specific HCPs are present in the sample from ELISA results, or which HCPs react immunologically with the antibodies used for detection. Therefore, regulatory authorities require that when using ELISA to detect residual HCP content, it is necessary not only to verify the accuracy, precision, sensitivity, and other parameters of the kit through methodological validation but also to demonstrate that the HCP antibodies used have broad reactivity—i.e., HCP antibody coverage.
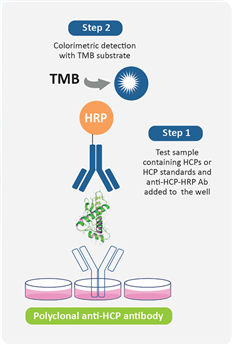
Figure 1: Detection Procedure of HCP ELISA Kit
When to Perform HCPs Antibody Coverage Verification?
The United States Pharmacopeia (USP) <1132> and European Pharmacopoeia (EP) 2.6.34. "HOST-CELL PROTEIN ASSAYS" categorize Host Cell Protein (HCP) detection methods into three types: commercial assay kits, product/process-specific methods, and platform methods. They also indicate that different ELISA kits are recommended for HCP detection at various stages of product development.
USP <1132> states that in the absence of a platform method, commercial assay kits can be used during the preclinical phase, Phase I clinical trials, and Phase II clinical trials. During Phase III clinical trials, process validation, and post-marketing of the product, due to the limitations of commercial universal HCP detection kits—such as insufficient antibody coverage—factors like cell type and process specificity should be considered, and either platform methods or product/process-specific methods developed for upstream processes should be adopted.
However, if it is necessary to continue using commercial assay kits after Phase II clinical trials, HCP antibody coverage verification must be conducted to assess whether the kits can still be used for quality control. It is generally recommended to perform the coverage verification at the end of Phase II clinical trials or before the start of Phase III clinical trials. At this stage, the production process has been finalized and there is relatively sufficient time for the verification.
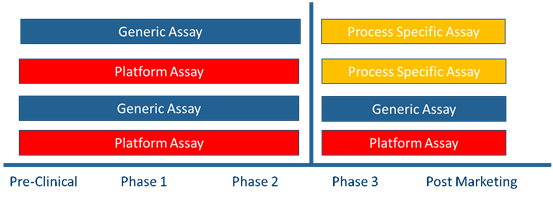
Figure 2 Recommendations for HCP Detection Methods at Different Stages of Product Development (USP <1132>)
Different Technical Approaches for HCP Coverage Verification
The traditional analytical method for coverage verification employs 2D Western blot (2D WB). Based on the principle that proteins differ in isoelectric point and molecular weight, proteins are separated on a gel using the SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) method. One of the gels is subjected to silver staining, while the other is transferred onto a PVDF (Polyvinylidene Fluoride) membrane. Western Blotting detection is then performed by combining the membrane with antibodies from the ELISA kit. However, due to its inherent methodological limitations and technical challenges, this method often struggles to comprehensively and accurately assess the coverage range of polyclonal antibodies for HCPs.
Limiting Factors of the 2D WB Method:
· Low loading capacity
· Pretreatment of samples damages the native epitopes of proteins
· The binding of HCPs to the PVDF membrane results in the loss of part of the detectable antibody information
· Difficulty in achieving complete alignment between the PAGE gel and the WB image
· Poor specificity, which significantly underestimates the actual antibody coverage
To address this challenge, Cygnus Technologies innovatively launched the Antibody Affinity Extraction (AAE?) technology in 2014. Its purpose is to enhance the sensitivity and coverage assessment of the total HCP mixture in cell culture harvests, as well as to detect the reactivity towards process-specific HCPs in downstream stages—these are usually the HCPs of greatest concern. Unlike 2D-PAGE and 2D-WB, whose sensitivity is limited by factors such as loading capacity, the sensitivity of AAE? is more than 100 times higher, as it can extract and concentrate large-volume samples.
Table 1 Comparison of AAE and 2D-WB Technical Routes for Coverage Verification
|
|
AAE |
2D-WB |
|
Sensitivity |
Can enrich HCPs at the milligram |
Due to the limitations of the WB method, the coverage rate is underestimated. |
|
High Sensitivity (>95%) |
Low Sensitivity (50-70%) |
|
|
Specificity |
High Specificity (>99.5%) with Non-Denaturing Treatment |
Low Specific Binding (50-80%) After Antigen Denaturation Treatment |
|
Coverage rate |
AAE+2D-PAGE 60-90% |
50-70% |
|
AAE-MS 70-95% |
||
|
Applicability |
Applicable both before and after purification |
Only suitable for samples before purification |
AAE Analysis Workflow
First, polyclonal antibodies are covalently immobilized on a chromatographic column. The column is then conditioned to prevent significant leaching of the antibodies and greatly reduce any non-specific binding. The original non-denatured HCPs sample is passed through the column to bind with the antibodies, followed by elution with acid. Through the processes of binding and elution, the HCPs sample is recycled on the column repeatedly until no additional HCPs are bound. All collected HCPs eluates are then pooled, subjected to buffer exchange, and concentrated back to the original sample volume, providing high-quality samples for subsequent analysis. After the HCPs samples are collected, subsequent analysis can be optionally performed via 2D-PAGE combined with silver staining or MS (Mass Spectrometry).
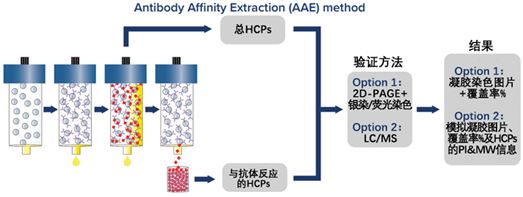
Figure 3 Recommendations for HCP Detection Methods at Different Stages of Product Development (USP <1132>)
Antibody Reactivity to Downstream HCPs and HCP Identification via AAE-MS?
The advantages of AAE? technology lie not only in its high sensitivity, but also in its robust predictive capability and broad application prospects. The coverage results obtained through AAE-MS? are higher and more authentic, enabling more accurate prediction of the performance of anti-HCP antibodies in ELISA. Moreover, the combination of AAE? with mass spectrometry (AAE-MS?) can identify HCPs in harvest materials that react with antibodies, and obtain information on the molecular weight and isoelectric point (pI) of the proteins. This makes it possible to assess individual HCPs that persist during the purification process and optimize downstream purification procedures.
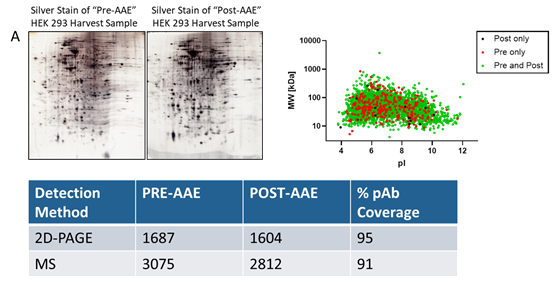
Notably, the AAE? technology performs excellently in detecting and demonstrating reactivity towards individual downstream HCPs. More importantly, mass spectrometry (MS) can identify the presence of potentially high-risk HCPs in samples and provide annotation information for these proteins. Through AAE? technology, these high-risk HCPs can be identified and quantified more accurately. These high-risk HCPs that persist through specific purification processes are crucial for patient safety, drug efficacy, and stability. Both pharmaceutical researchers and regulators can clearly understand the residual status of a specific HCP in the process via this technology, thereby providing strong support for drug research and development as well as production.
Table 2 High-Risk HCPs in CHO Cells
|
High-Risk Proteins(CHO) |
PRE |
F550 |
F550-1 |
pl |
MW |
|
78 kDa glucose regulated protein (BiP, HSPA5) |
N |
Y |
Y |
5.07 |
72379.1 |
|
Cathepsin B(CTSB) |
Y |
Y |
Y |
5.73 |
35646.9 |
|
Cathepsin D |
Y |
Y |
Y |
6.54 |
44110.9 |
|
Cathepsin E |
N |
N |
N |
4.61 |
42726.4 |
|
Clusterin |
Y |
Y |
Y |
5.58 |
51557.5 |
|
Glutathione S transferase P |
N |
Y |
Y |
7.64 |
23638.2 |
|
Matrix Metalloproteinase 19 |
Y |
Y |
Y |
7.71 |
58942 |
|
Monocyte chemoattractant protein 1 (MCP-1) |
Y |
Y |
Y |
9.32 |
15858.4 |
|
Peptidyl-prolyl cis-trans isomerase |
Y |
Y |
Y |
9.59 |
23634.4 |
|
…. |
|
|
|
|
|
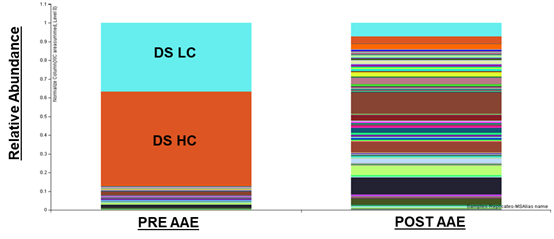
圖5 AAE對藥物原液中HCPs的富集
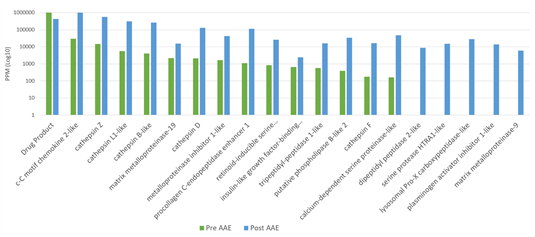
Figure 6 Enrichment of HCPs in Drug Bulk Drug Substance by AAE
In conclusion, the Antibody Affinity Extraction (AAE?) technology, with its excellent performance and broad application prospects, is gradually becoming the new standard for Host Cell Proteins (HCPs) analysis in the biopharmaceutical field. It can not only overcome the limitations of traditional methods, but also achieve significant improvements in sensitivity, predictive ability, and application depth. With the continuous development and improvement of the technology, it is believed that AAE? will contribute to the research and development and production of more drugs in the future, and help bring safer and more effective treatment solutions to patients.
Supporting Literature
[1] USP <1132> Residual Host Cell Protein Measurement in Biopharmaceuticals.
[2] USP <1132.1> Residual Host Cell Protein Measurement in Biopharmaceuticals by Mass Spectrometry.
[3] EP-2.6.34. Host-Cell Protein Assays.
[6] Antibody Affinity Extraction (AAE?) Empowers HCP Identification by Mass Spectrometry.


 京公網安備 11010802028692號
京公網安備 11010802028692號